As part of National Science Week 2019, we are highlighting some of the high-impact research being carried out by AINSE scholars. In the Biotechnology and Biomedical Sciences research theme, Flinders University student and AINSE PGRA scholar Melanie Fuller is investigating how gold nanoparticles could be used in combination with antibiotics to combat multi-resistant bacterial infections.

Understanding how antibiotics and gold nanoparticles affect bacterial membranes
Melanie Fuller, Jakob Andersson, Kathleen Wood, Stephen Holt, Ingo Kӧper
Multi-resistant bacterial infections are becoming more prevalent worldwide, with current estimations predicting 10 million deaths by 2050.
In an attempt to understand how different antibiotics affect the cell membrane, the antibiotic Colistin has been explored with a gram negative membrane model. Using tethered lipid bilayers, the interaction of Colistin with the membrane’s surface can be explored by using Neutron Reflectometry. The effect of adding gold nanoparticles prior to exposing the membrane to Colistin is also being explored.
“This research will provide a better understanding on how the combination of the particles and antibiotic are disrupting the membrane, and may assist in the development of new antibiotics which target the bacterial membrane. “
Our previous experiments found that Colistin disrupts the structure of the lipid membrane. This has been confirmed in Small Angle Neutron Scattering (SANS) experiments where Colistin was added to lipid vesicles. Prior to the addition of Colistin, the vesicles were observed to have a unilamellar structure with no Bragg peaks present (as shown in Figure 1). However, after the addition of Colistin, a more ordered structure was observed.
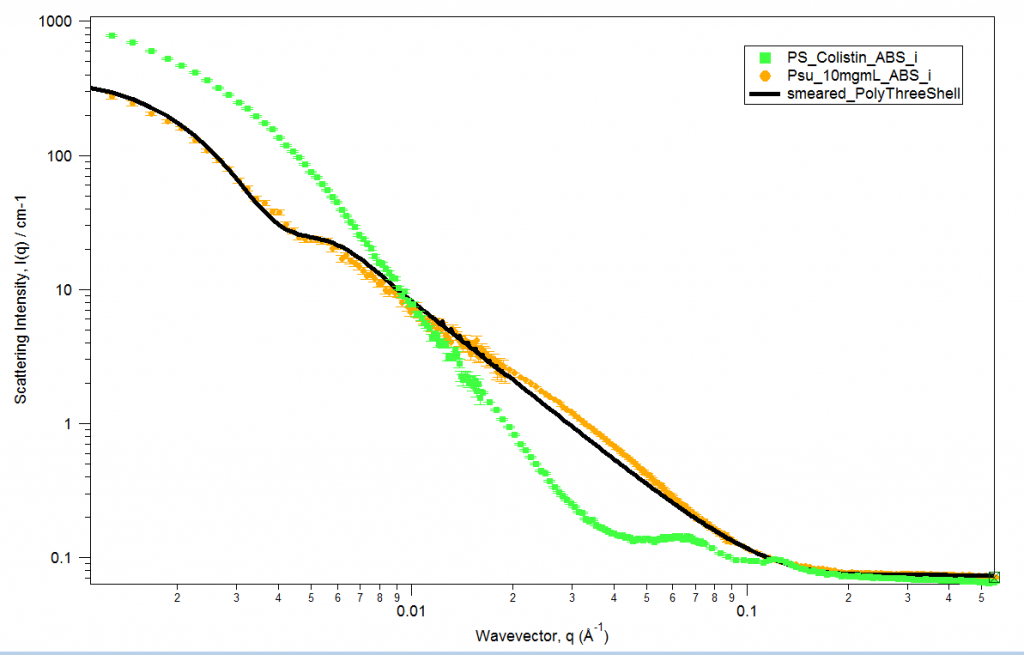
The ordered structure was determined to be a multilamellar structure with Bragg peaks occurring at q=0.064/A and q=0.107/A. The first peak also had a calculated d-spacing of 58/A and the secondary peak was attributed to a second order reflection. Thus the increased scattering intensity at low q, as well as the intensity plateau, suggests the addition of Colistin forms larger particles or vesicle aggregates (Andersson et al., 2018).
In our most recent work we have begun to explore the effects on the membrane structure of pre-treating gram-negative membranes with cationic gold nanoparticles prior to the addition of Colistin. Initial results using Electrochemical Impedance Spectroscopy (EIS) have shown that the addition of cationic gold nanoparticles prior to the addition of Colistin reduces the membrane resistance and prevents an increase in capacitance. This suggests that the nanoparticles are likely causing small defects in the membrane that allow Colistin to solubilise the lipopolysaccharides at the defect site, causing irreversible damage to the membrane.
The next step in this research is to use neutron reflectometry with a tethered lipid bilayer to model which parts of the membrane the gold nanoparticles and Colistin are affecting (Figure 2).
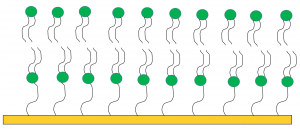
This research will provide a better understanding on how the combination of the particles and antibiotic are disrupting the membrane, and may assist in the development of new antibiotics which target the bacterial membrane.
Acknowledgements:
The authors acknowledge the Australian Institute of Nuclear Science and Engineering for the PGRA research funding.
References:
Andersson, J, Fuller, M A, Wood, K, Holt, S A & Köper, I 2018, ‘A tethered bilayer lipid membrane that mimics microbial membranes’, Physical Chemistry Chemical Physics, vol. 20, no.18, pp. 12958–12969.
