As part of National Science Week 2019, we are highlighting some of the high-impact research being carried out by AINSE scholars. In the Biotechnology and Biomedical Sciences field, Curtin University student and AINSE PGRA scholar Mitchell Klenner is working to develop techniques to synthesize new diagnostic nuclear medicines.

Unlocking the potential of rhenium for improved nuclear medicines
Mitchell Klenner, Giancarlo Pascali, Max Massi & Benjamin Fraser
Radiotracers are commonly used to investigate biochemical systems in living organisms and are routinely employed as nuclear medicines to diagnose cancers and other disease pathologies.
To synthesise nuclear medicines, a drug or biomolecule needs to be radiolabelled with a radioisotope. The radioisotope then emits detectable radiation from the site of the tumour or organ where the radiotracer has accumulated. However, many disease states remain untreated by nuclear medicines—not necessarily because new molecules are needed to target these diseases, but also because of the immense difficulty required to radiolabel these molecules.
The radiolabelling process needs to be performed as time-efficiently as possible, since radioactive decay during the time of synthesis will result in less radioactivity being available for imaging following administration of the radiotracer to the patient. Thus the discovery of new radiolabelling methods is critical: not only for the improved synthesis of currently-existing nuclear medicines but also to enable the synthesis of new radiotracers that could eventually become the nuclear medicines needed to diagnose untreated disease states.
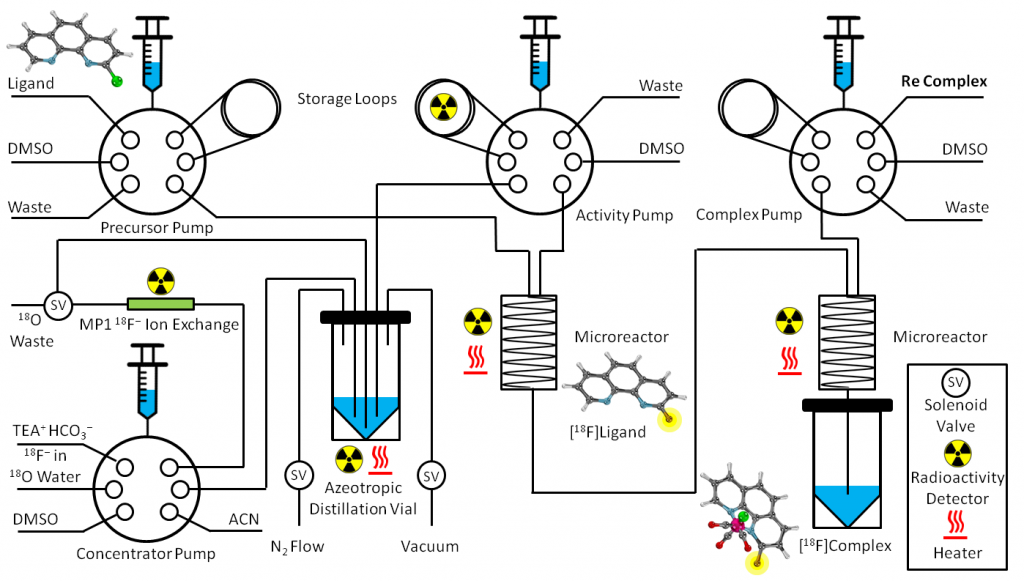
Herein we describe a novel radiolabelling method used to improve the molecular incorporation of fluorine-18 (Klenner et al., 2017a), the most commonly-employed radioisotope used in the most sensitive of nuclear imaging techniques, positron emission tomography (PET) imaging. We tested this method repeatedly using an automated synthesis module, as shown in Figure 1, which enabled us to repeat reactions under the same conditions while changing only the temperature as a variable, thus revealing temperature-dependent trends within our method.
“This opens new avenues for developing Alzheimer’s PET medicines that can now be investigated.”
The method involves first and foremost the complexation of a bidentate molecule to a source of rhenium(I), in our case using pentacarbonylchlororhenium(I) chloride (Klenner et al., 2017b), which activates the bidentate molecule via electron withdrawal as suggested by our nuclear magnetic resonance (NMR) spectrometry data. Fluorine-18 radiolabelling of the rhenium complex then results with high radiochemical yield (RCY) up to 130°C, as shown by the orange chromatogram in Figure 2. At higher temperatures, the radiolabelled complex then dissociates in order to liberate the radiolabelled ligand in considerable RCY, as evidenced by the cyan line of Figure 2.
Perhaps the most impressive aspect of this method is that the radiolabelled ligand, [18F]6-fluoro-2,2’-bipyridine, was unable to be synthesised under any of our radiolabelling conditions until complexed with the source of rhenium. Figure 3 shows the overall trend of forming the fluorine-18 labelled complex in greater RCY as temperature increases, as shown by the red line (pink crosses), until it eventually dissociates at temperatures greater than 130°C to free the radiolabelled ligand of interest, as shown by the blue line (cyan crosses).
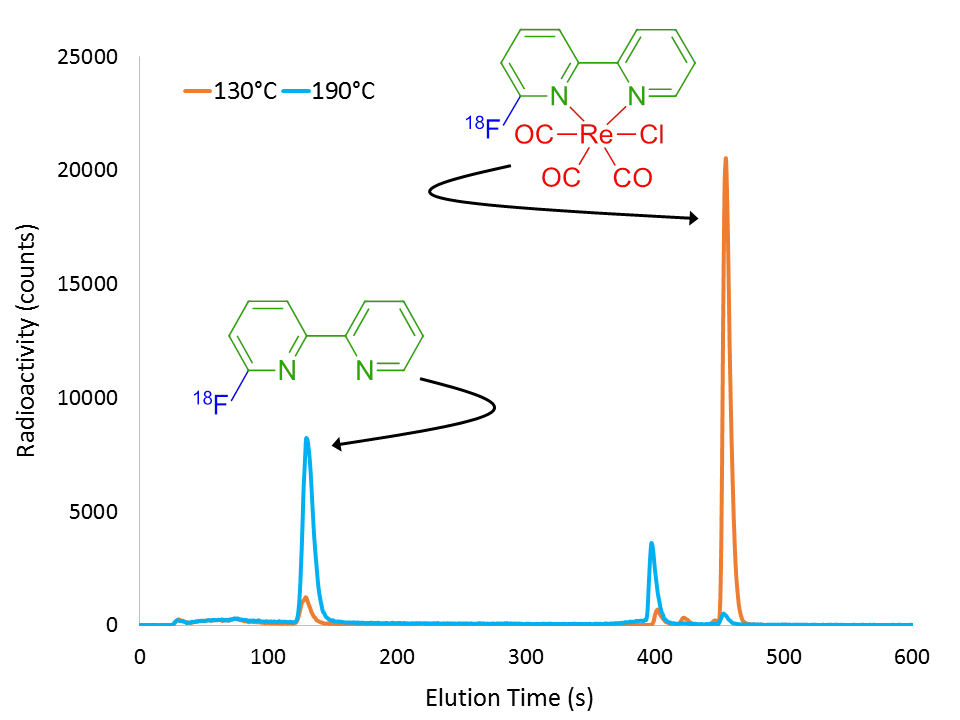
Figure 2 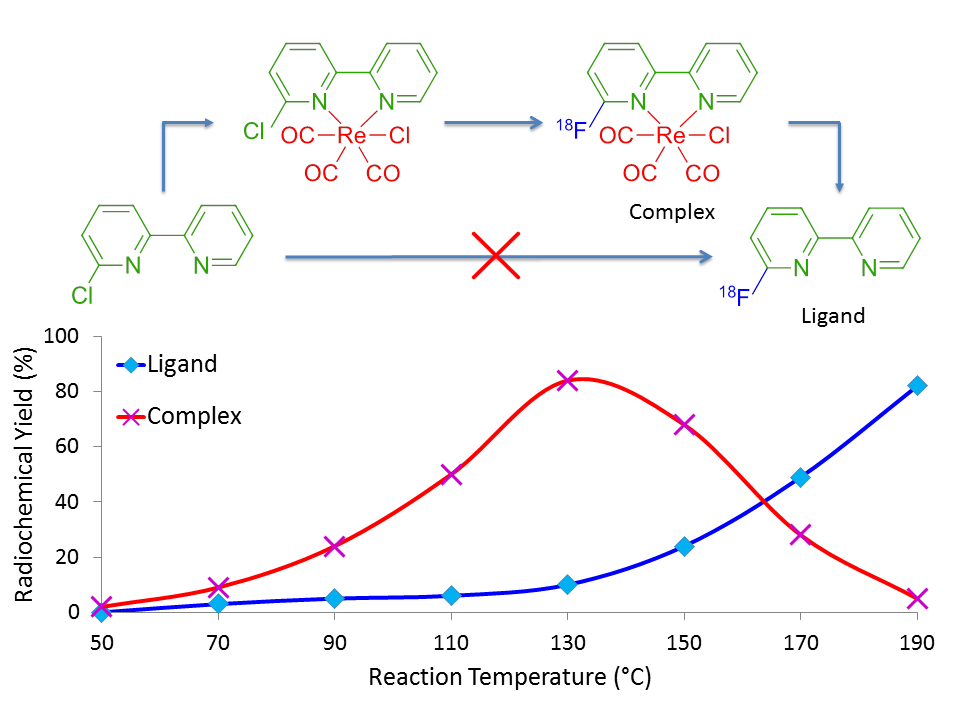
Figure 3 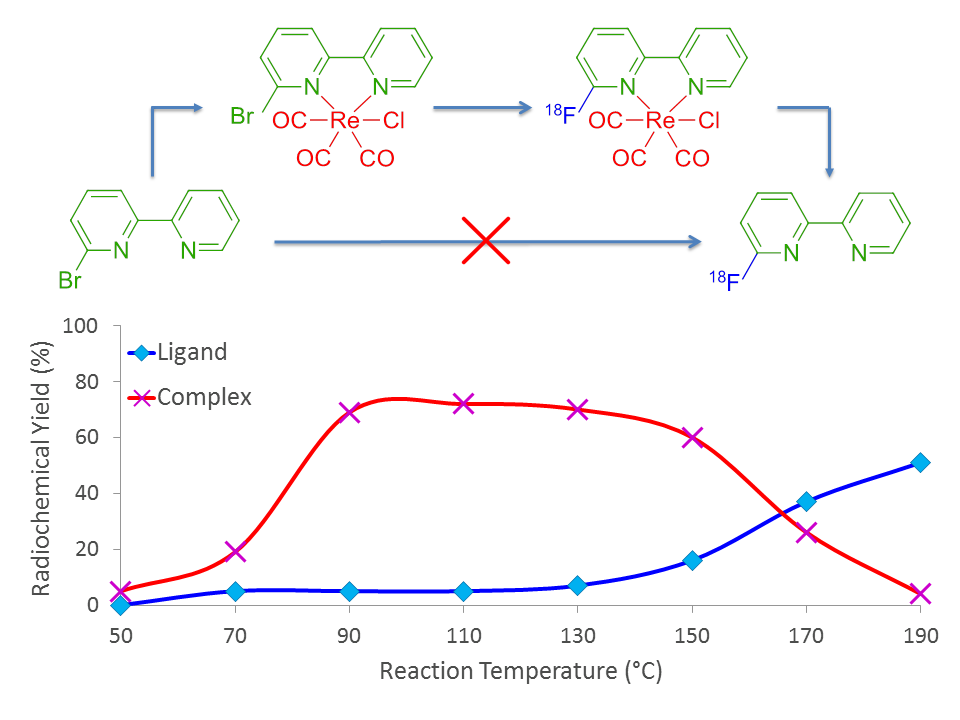
Figure 4 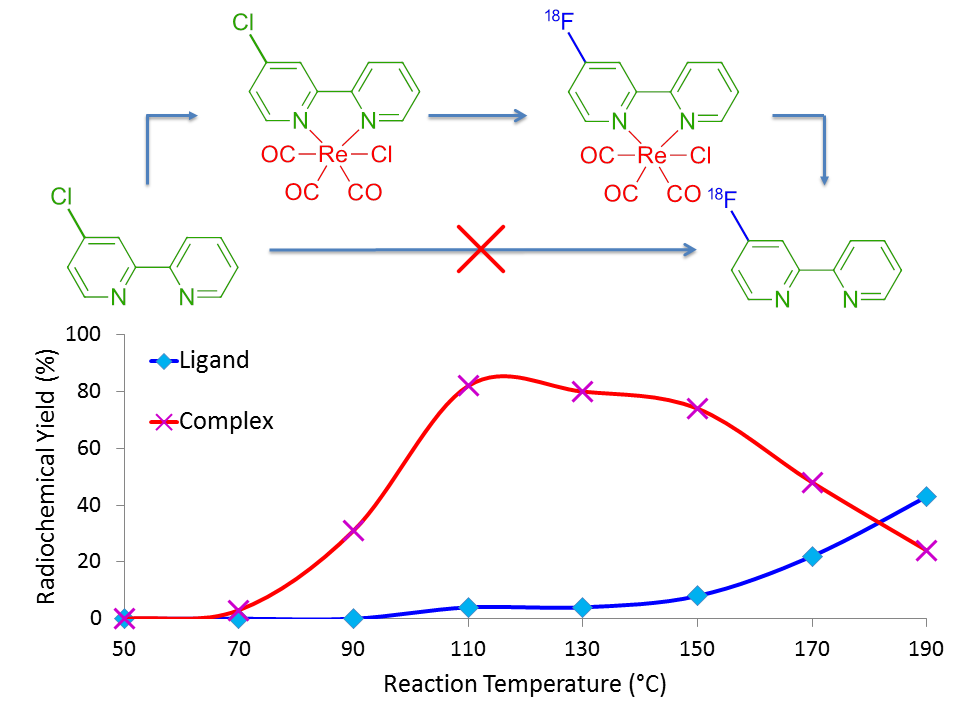
Figure 5 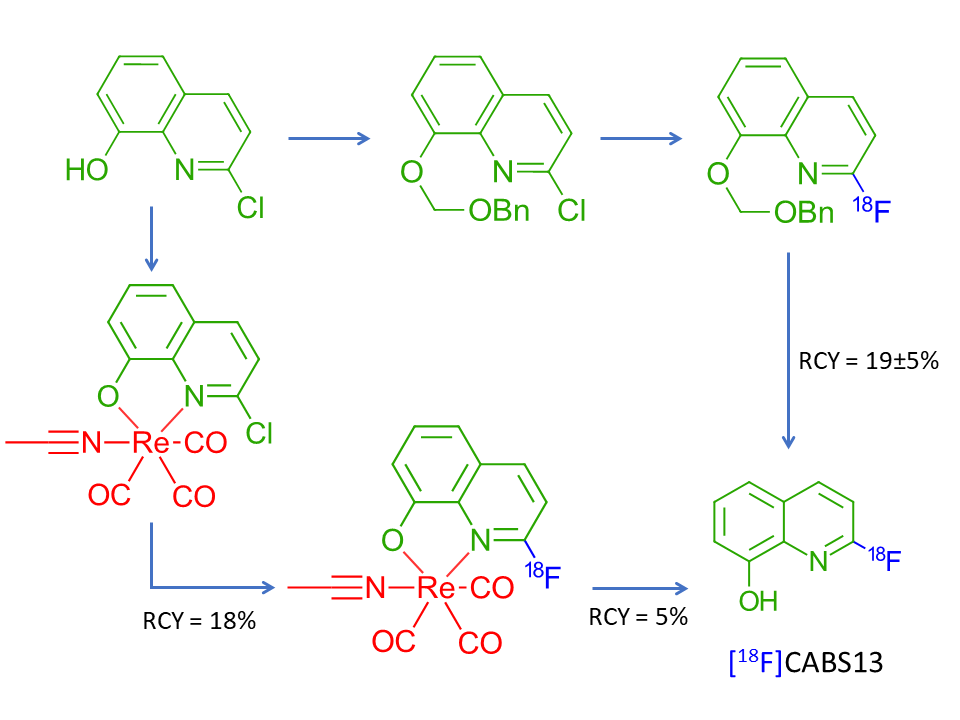
Figure 6
Following this discovery, we pursued a systematic investigation to determine the limitations of radiofluorination and discovered that we could use this method to substitute our fluorine-18 radioisotope for different atoms, as shown in Figure 4 (substituting for bromine instead of chlorine), and could radiolabel in different positions of the molecule, as shown in Figure 5.
Based on these observations we attempted to apply this novel rhenium complexation-dissociation strategy for the improved synthesis of [18F]CABS13, a PET medicine used in the imaging and disaggregation of amyloid-β plaques characteristic of Alzheimer’s disease (Vasdev et al. 2012, Liang et al. 2015a). Preliminary experiments have returned 18% RCY of the rhenium complex and 5% RCY of the [18F]CABS13 ligand versus the 19±5% RCY attained though multistep radiosynthetic processes in literature, as shown in Figure 6.
Excitingly, this method was able to synthesise the medicinal compound [18F]5-fluoro-8-hydroxyquinoline, which is an alternative to [18F]CABS13 that has been shown to disaggregate amyloid-β plaques more effectively in cell experiments, though remained unable to be radiosynthesised until now (Liang et al. 2015b). This opens new avenues for developing Alzheimer’s PET medicines that can now be investigated.
Acknowledgements:
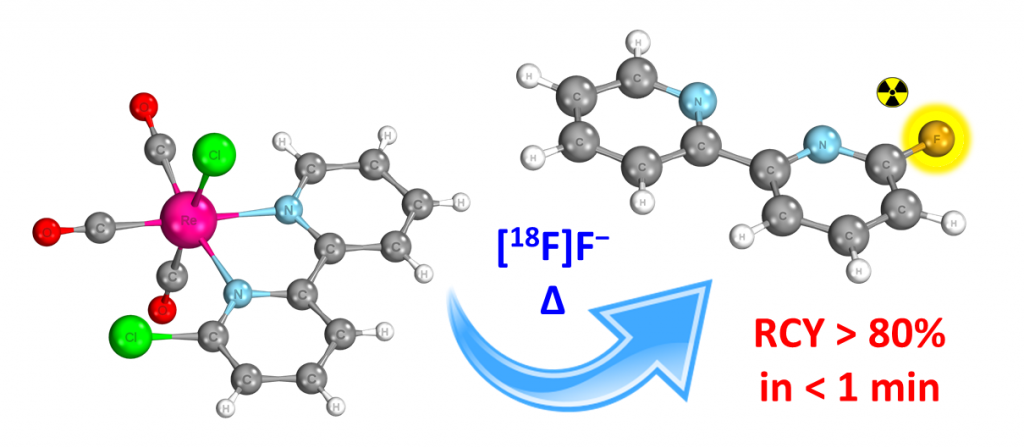
The discovery of this novel rhenium complexation-dissociation approach is incredibly exciting news which could not have made possible without the hard work, expertise and collaboration of others. Foremost is the intellectual support and supervision provided from Dr. Giancarlo Pascali, Dr. Max Massi and Dr. Benjamin Fraser. The appreciation is extended also to Dr. Helen Maynard-Casely and Jack Clegg, for their collaboration at the Australian Synchrotron (refer to crystal structure in Figure 7), as well as Bo Zhang and Dr. James Howard for their aid in the radioactive and non-radioactive syntheses respectively. Finally, we also express our immense gratitude to AINSE, not only for the generous funding which bought about the success of this project, but also for their compassionate and encouraging support along the way.
References:
Klenner, M A, Pascali, G, Zhang, B, Sia, T R, Spare, L K, Krause-Heuer, A M, K.L. Aldrich-Wright, J, Greguric, I, Guastella, A, Massi, M & Fraser, B H 2017a, ‘A fluorine-18 radiolabeling method enabled by Rhenium(I) complexation circumvents the requirement of anhydrous conditions’, Chemistry: A European Journal, vol. 23, pp. 6499–6503.
Klenner, M A, Pascali, G, Zhang, B, Sia, T R, Spare, K L Aldrich-Wright, J, Greguric, I, Guastella, A, Massi, M & Fraser, B H 2017b, ‘Effect of Re(I) complexation in the radiosynthesis of prospective [18F] fluorine labelled PET/optical probes’, Journal of Labelled Compounds and Radiopharmaceuticals, vol. 60, p.S65.
Vasdev, N, Cao, P, Van Oosten, E M, Wilson, A A , Houle, S, Hao, G, Sun, X, Slavine, N, Alhasen, M, Antich, P P, Bonte, F J & Kulkarni, P 2012, ‘Synthesis and PET imaging studies of [18F]2-Fluoroquinolin-8-ol ([18F]CABS13) in transgenic mouse models of Alzheimer’s disease’, MedChemComm, vol. (3), pp. 1228–1230.
Liang, S H, Holland, J P, Stephenson, N A, Kassenbrock, A, Rotstein, B H , Daignault, C P, Lewis, R, Collier, L, Hooker, J M & Vasdev, N 2015a, ‘PET neuroimaging studies of [18F]CABS13 in a double transgenic mouse model of Alzheimer’s disease and nonhuman primates’, ACS Chemical Neuroscience, vol. 6, pp. 535–541.
Liang, S H, Southon, A G, Fraser, B H, Krause-Heuer, A M, Zhang, B, Shoup, T M, Lewis, R, Volitakis, I, Han, Y, Greguric, I, Bush, A I & Vasdev, N 2015b, ‘Novel fluorinated 8-hydroxyquinoline based metal ionophores for exploring the Metal Hypothesis of Alzheimer’s disease.” ACS Medicinal Chemistry Letters, vol. 6, pp. 1025–1029.
