By Georgia Barrington-Smith
Beneath abandoned mine sites, a silent chemical threat lingers.
When sulfide-rich rocks are exposed to air and water during mining, they trigger acid mine drainage—acidic runoff that releases toxic elements like arsenic (As) and antimony (Sb) into surrounding soil and water.This contamination can persist for decades after mining operations cease, threatening the health of both people and delicate ecosystems.
Fortunately, there is a naturally occurring mineral, jarosite, which forms in these acidic, sulfur-rich environments and which may have the potential to trap arsenic and antimony by binding them to its surface. This ‘trapping’ may influence the distribution of these toxic elements. Scientists are continuing to investigate the effectiveness of this naturally occurring trap, as well as whether the presence of jarosite itself would reduce or increases the environmental risks.
Toxic Twins: Arsenic and Antimony
Arsenic (As) and antimony (Sb) are naturally occurring metalloids often found alongside metal-rich rocks in the Earth’s crust. While useful in technologies such as electronics and flame retardants, both elements become highly toxic when released into the environment at elevated concentrations.
A key process influencing their mobility and environmental impact is sorption, in which one substance can effectively sequester large quantities of these metalloids. The sorption process plays a major role in controlling whether elements like As and Sb stay bound to solid materials or leach into groundwater and soil.
While arsenic and antimony have been studied separately on iron minerals, their combined behaviour on jarosite is not yet understood.
Do these metals compete for identical binding sites, or do they facilitate each other’s attachment?
Understanding this relationship is critical for predicting how mobile, and thus how dangerous, these elements are over time.
Putting jarosite to the test: Can it trap arsenic and antimony?
A team of researchers led by Dr. Niloofar Karimian, a Mineral Resources Scientist at CSIRO, in collaboration with Monash University, Southern Cross University, and with the support of the AINSE Early Career Researcher Grant (ECRG), investigated how As and Sb bind to jarosite in mining-impacted environments.
They examined how As(V) and Sb(V) (the oxidised forms of and Sb) bind to jarosite in both single-metal and mixed-metal systems.
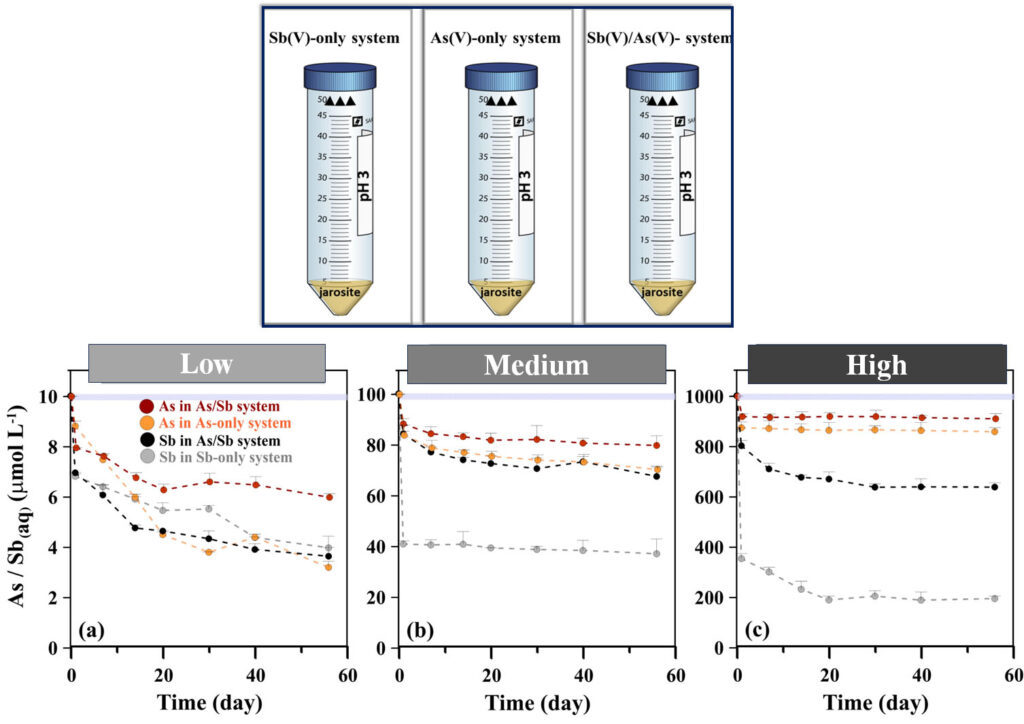
The team conducted lab-based sorption experiments using synthetic jarosite aqueous solutions. These solutions were exposed to As(V) and Sb(V), both individually and combined, at pH 3 to reflect the acidic conditions commonly found in mine waste environments.
Three concentrations were tested: low (10 µmol/L), medium (100 µmol/L),and high (1000 µmol/L), to assess how varying contaminant levels affected metal uptake over time. The mixtures were then agitated on orbital shakers for up to 56 days to simulate extended environmental exposure, allowing the researchers to observe both initial binding and longer-term reactions that might affect metal stability or release.
Using synchrotron spectroscopy to reveal metal behaviour
To determine precisely how the metals were binding to jarosite at the molecular level, the team used the multi-edge X-ray Absorption Spectroscopy (XAS) beamline at ANSTO’s Australian Synchrotron. This powerful technique provided insight into bonding types, chemical structure, and local atomic environments, shedding light on how securely (or loosely) the toxic metals were bonded to jarosite.

By combining long-term sorption experiments with cutting-edge synchrotron analysis, the researchers could measure both how much metal was retained and the precise mechanism by which it was bound to the mineral surface.
What was uncovered from the spectroscopy analysis?
- Jarosite binds Sb(V) more strongly than As(V)
When Niloofar and her collaborators tested As(V) and Sb(V) separately (in single-metal systems), they found that Sb(V) attached more strongly to the mineral jarosite than As(V).
However, when both metals were present (in dual-metal systems), they began to compete for the same binding sites on the jarosite surface. As a result, neither metal attached as effectively, reducing their binding capabilities by about 50%.
This competition suggests that in real-world contaminated environments, where multiple pollutants often coexist, each element can influence the other’s behaviour and mobility.
- Antimony forms new mineral-like structures
Instead of attaching to the surface of the jarosite, Sb(V) actually caused new tiny solid materials, called surface precipitates, to form on top of the jarosite. These precipitates were made up of complex clusters of Sb and oxygen (O2) atoms called polynuclear Sb oxide species.
This finding indicates that Sb(V) doesn’t passively bind to the jarosite, but instead actively changes the mineral surface, creating new solid phases that have not been seen before in this context.
This newly discovered mechanism for Sb(V) behaviour may influence our current understanding of its stability and mobility in contaminated environments.
- Arsenic binds differently depending on Sb levels
When alone, or with just a trace of Sb(V), As(V) attached directly onto jarosite. However, this behaviour changed when Sb(V) levels increased: As then shifted its attachment from jarosite to the oxide layer formed by Sb(V).
This reveals a subtle yet significant interaction, whereby rather than competing for the same binding sites, Sb(V) modifies the mineral environment, prompting As(V) to adapt accordingly.
It underscores how contaminants rarely act in isolation, instead influencing each other’s behaviour and transport in complex and unexpected ways.
These finding challenges previous assumptions by uncovering a novel mode of interaction among co-occurring pollutants, enhancing our ability to predict environmental risks and improve management strategies for contaminated sites.
A step toward better risk assessment models
Dr. Karimian’s research deepens our understanding of how toxic metals behave and co-behave in contaminated environments.
By uncovering how As and Sb interact with jarosite, this study helps:
- Predict pollutant behaviours in mining-impacted areas,
- Improve models of contaminant mobility, and
- Guide the design of better long-term remediation strategies.
While strong mineral binding can reduce short-term risks, environmental shifts, like changes in pH, may eventually destabilise these bonds, releasing toxic elements back into ecosystems.
Through a combination of cutting-edge synchrotron techniques and detailed lab-based experiments, Niloofar’s research lays the groundwork for future studies exploring how pollutants interact with minerals under real-world conditions.
AINSE is proud to support early-career researchers like Dr. Karimian through grants and programs that promote impactful science.
Explore AINSE scholarships at ainse.edu.au/scholarships and view more research features at ainse.edu.au/research.
Coming up in August: Research Spotlights – AINSE Edition!
Thank you for joining us for Geochemistry July!
Coming up in August, we’re excited to present a special edition of our Research Spotlights.
In celebration of National Science Week, we’re taking a trip down memory lane, revisiting some of our past Research Spotlights in a fun-filled week of science, every day!
Stay tuned as we reveal a daily theme across the week!
Follow ainse_ltd on Instagram, Facebook, Threads and LinkedIn to keep up to date with upcoming events and research spotlights.
