By Georgia Barrington-Smith
In the quest to unravel one of life’s greatest mysteries—how it all began—scientists are looking beyond our planet, to the vastness of space, in search of the molecular seeds that might have sown life on Earth.
One key stop on that journey is Titan—Saturn’s largest moon.
For nearly two decades, NASA’s Cassini spacecraft orbited Saturn, collecting stunning data about the planet’s rings, moons, and magnetic environment. Accompanying it was the European Space Agency’s Huygens probe, which parachuted down to Titan’s surface, becoming the first spacecraft to land on a world in the outer Solar System.
What it found was astonishing!
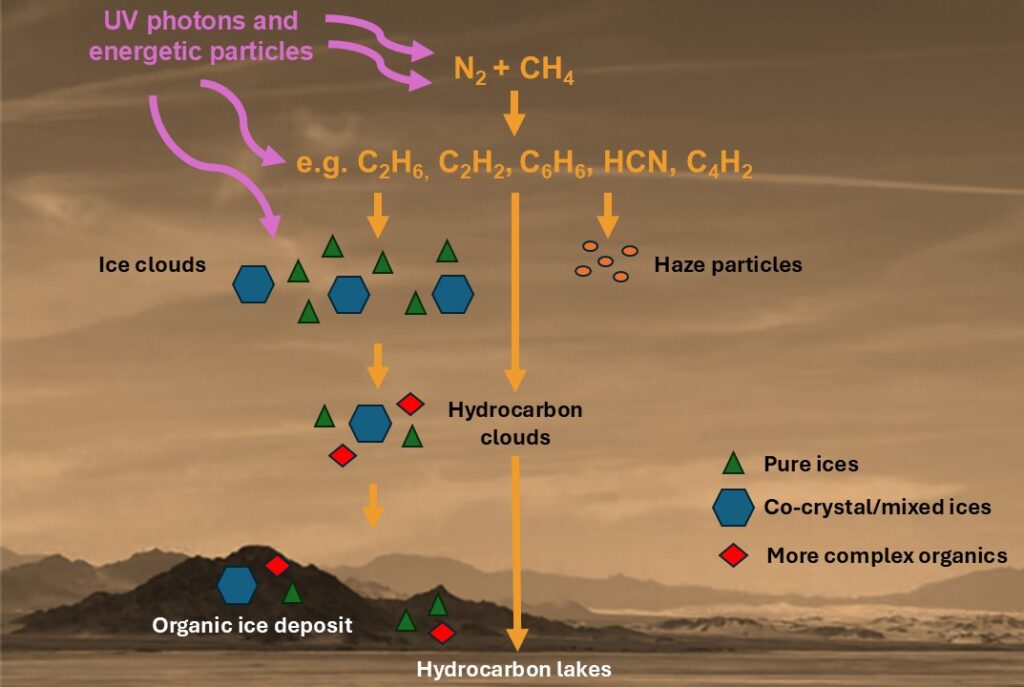
This mission revealed that Titan isn’t just a frozen, foreign world, it’s a chemical treasure trove. Its atmosphere contains more than twenty complex organic molecules. Among the most intriguing are nitrogen-rich compounds that could serve as stepping stones in the formation of prebiotic molecules, the very kind that may have paved the way for microbial life on early Earth.
How Titan’s co-crystals could hold clues to life’s beginnings
New research suggests that Titan’s icy temperatures and dense, organic-rich atmosphere create ideal conditions for the formation of co-crystals: solid structures made up of two or more molecules arranged in a fixed, orderly pattern (Cable et al. 2021). These ordered molecular arrangements provide a scaffold that allows close interactions between complex compounds, providing stepping stones toward biologically relevant molecules.
Therefore, studying these co-crystals, which can stabilise these fragile complex molecules, is critical for understanding how prebiotic chemistry (the chemical processes that generate life’s building blocks, such as amino acids and nucleotides) develops and survives in harsh extraterrestrial environments.
One compound of particular interest is pyridine, a nitrogen-containing aromatic compound thought to exist in Titan’s atmosphere. Pyridine interacts with hydrocarbons like acetylene to create stable co-crystals, which are believed to act as precursors to nucleobases (the building blocks to DNA and RNA). This makes pyridine a promising candidate for exploring how co-crystal formation might contribute to prebiotic chemistry on Titan.
Larissa investigates Titan’s prebiotic potential
Larissa Lopes Cavalcante, an AINSE PGRA scholar, in collaboration with ANSTO and the University of Otago, is investigating pyridine-based co-crystals and their potential role in forming prebiotic molecules. Her research focuses on combining pyridine with key molecules found in Titan’s atmosphere, like acetylene, diacetylene, ethane, and acrylonitrile.
By using techniques such as X-ray diffraction, infrared and Raman spectroscopy, mass spectrometry, and computational modelling, Larissa aims to characterise these co-crystals and uncover the pathways that might lead to life’s building blocks.
Simulating Titan’s atmosphere to explore prebiotic molecules
One focus of Larissa’s research is the pyridine: acetylene system, previously shown to form stable co-crystals under Titan-like conditions. She studied the reactivity of this mixture under such conditions to determine if it can give rise to more complex organic molecules.
To test the systems reactivity, the team exposed both amorphous (disordered) and crystalline (ordered) pyridine: acetylene ices to vacuum-ultraviolet (VUV) radiation, simulating the high-energy conditions in Titan’s atmosphere (Lopes Cavalcante et al. 2024).
Under VUV exposure, pyridine and acetylene reacted to form nitrogen-containing polycyclic aromatic hydrocarbons (NPAHs)—complex molecules that may serve as intermediates in the formation of life-related compounds.
Interestingly, the crystalline co-crystal form showed lower reactivity than its amorphous counterpart. This greater chemical stability makes it better equipped to withstand Titan’s extreme conditions. Maintaining the stability of the co-crystal is crucial for preserving pyridine, a key molecule in prebiotic chemistry. Once the co-crystal reaches Titan’s surface, pyridine could be released and become available to interact with other chemical species present there. Even more intriguing is the possibility that these surface molecules could be transported to Titan’s subsurface ocean, where conditions may allow for more complex, Earth-like prebiotic chemistry to unfold.
The role of pyridine: diacetylene in Titan’s prebiotic chemistry
Larissa and the team also explored the pyridine: diacetylene system for its potential role in Titan’s prebiotic chemistry.
Diacetylene, a hydrocarbon abundant in Titan’s atmosphere, is notoriously unstable and prone to polymerisation. Therefore, to safely study it, researchers at ANSTO’s Australian Centre for Neutron Scattering (ACNS) developed an advanced gas delivery system for neutron diffraction experiments. This innovative system enabled researchers to securely handle diacetylene and accurately deliver it without premature reactions, establishing a foundation for future studies on the formation of co-crystals involving highly flammable, toxic, and reactive molecules at Titan-relevant temperatures (-180°C). A major challenge with diacetylene is its tendency to polymerize upon contact with oxygen, forming unstable and potentially explosive materials. One of the most significant improvements introduced by this new gas delivery system is its ability to minimize that risk—while handling diacetylene still requires caution, the system offers far greater control and safety, drastically reducing the likelihood of hazardous reactions.
Using the WOMBAT powder diffractometer at ACNS, along with X-ray diffraction (University of Sydney) and Raman spectroscopy (Jet Propulsion Laboratory, Caltech), the team determined the crystal structure of pure diacetylene under Titan-like cryogenic conditions.
After characterising diacetylene in isolation, the team started a new series of experiments to investigate its interactions when combined with pyridine. The results revealed the formation of a new crystalline phase. This newly identified solid crystal structure corroborates earlier infrared spectroscopic data. It may represent a novel class of co-crystal pertinent to Titan’s chemistry, suggesting that, if such co-crystals exist on Titan, they could influence the storage, preservation, or synthesis of complex organic molecules in its environment.

Unexpected behaviour with ethane and acrylonitrile
In addition to studying pyridine mixed with acetylene and diacetylene, Larissa and her collaborators also investigated what happens when pyridine is combined with ethane and acrylonitrile.
Surprisingly, instead of forming co-crystals, these combinations triggered a phase transition in pyridine, a change in its physical state or structure. This unexpected behaviour adds complexity to the understanding of Titan-like chemical systems and highlights the diverse ways organic molecules may interact in such extreme environments.
Looking ahead: Titan’s chemistry and the ongoing search for the origins of life
Together, Larissa and the team’s findings not only deepen our understanding of Titan’s unique environment but also shed light on the broader question of whether life could have started elsewhere in the solar system.
Pyridine-based mixed ices show promise to form the building blocks of life, such as nucleobase precursors and other key organic molecules. On the other hand, the stability of co-crystals in Titan’s harsh conditions suggests they could protect organic compounds for long periods, potentially allowing them to be transported to places where prebiotic chemistry could take place.
This research is especially relevant for upcoming missions like NASA’s Dragonfly, which prepare to explore Titan’s surface investigating its chemical and astrobiological potential for life.
AINSE are proud to spotlight Larissa for her stellar work!
If you, like Larissa, are curious about studying the chemistry of our solar system, visit ainse.edu.au/scholarships to see how AINSE can support you.
Join us on your next stop for this lunar journey as we spotlight Jay Archer’s groundbreaking research on the effects of lunar space radiation.
While you wait, read some more out of this world research, at ainse.edu.au/research-spotlights/ and explore the rest of our catalogue.
Follow ainse_ltd on Instagram, Facebook, Threads and LinkedIn to keep up to date with upcoming events and research spotlights.
